How Many Electrons Are in the Outer Shell of Magnesium
It needs two electrons to complete its octet so it uses its two s-electrons to achieve this. A magnesium atom has the highest number of electron energy levels.
Chem4kids Com Magnesium Orbital And Bonding Info
Magnesium acquires a full octet by losing 2 electrons and emptying out its outermost shell.

. When these electrons are lost a magnesium ion Mg 2 is formed. A magnesium atom has the highest number of electrons per unit. Magnesium atoms have two electrons in the outermost shell and chlorine atoms have seven.
How many electrons. How many electrons would be in the outer shell of magnesium once it becomes a ion. 8 How many protons neutrons and electrons does mg2 have.
11 How many protons and electrons does ca2 have. First know that a valence electron is an electron in an outer shell. The compound magnesium chloride would contain_____ 1 magnesium and 2 chlorine.
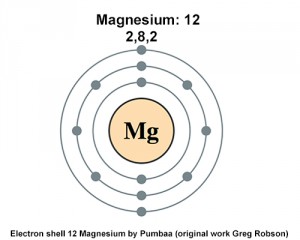
You can see two electrons in shell one eight in shell two and two more in shell. Sodium has one valence electron. There are 2 electrons in outer shell of Magnesium on periodic table.
Magnesium needs to lose two electrons to get a full outer shell. Magnesium has two electrons in its outer shell as it is in group 2 of the periodic table which it loses so its outer shell is full 28 and it has a charge of 2 as it still has 12 protons positive charges and now has only 10 electrons negative charges. 8 because it will lose its 2 valence electrons since theres TWO chlorine ions.
How much magnesium is present in your body. Why does sodium only have 1 valence electron. It can either share these two outer electrons or lose them.
13 How many neutrons does ca2 have. Therefore 2 electrons in its outer shell. The electron configuration would be 1s2 2s2 2p6 3s2.
In this case magnesium bonds with two chlorine Cl atoms which each need one electron. The electronic configuration of Magnesium is 2 8 2. How Many Valence Electrons Does Magnesium 2 Have.
Magnesium has two electrons in its outer shell as it is in group 2 of the periodic table which it does not have so its outer shell is clean 2- and has no electrons negative charges. Magnesium has a total of 12 electrons 2 in the innermost shell 8 in the second shell and two electrons in its valence shell third shell. 9 What is the proton number of calcium.
Group of answer choices. Joette Answeregy Expert. Magnesium is in Group 2.
The chemical symbol for Magnesium is Mg. Magnesium atoms has two electrons in their outer third shell and chlorine atoms have seven electrons in their outer shell. It has two electrons in its outer shell.
Oxygen atomic number 8 requires how many additional electrons to fill its outer electron shell. The full outer shell of magnesium is the 4s 2 3d 10 electronic configuration. The electron arrangement of magnesium is 282 meaning it has two outer electrons.
With the additional electron chlorine has a filled shell and magnesium loses two electrons. The atomic number of calcium is 20 and its electronic configuration is 2 8 8 2 which shows that the outermost. How many electrons are in the outer shell of magnesium Answer.
10 What is the electron number of calcium. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. How many electrons are in the outer shell of magnesium in the compound magnesium chloride MgCl 2.
Magnesium Mg It has two electrons in its outer shell. Magnesium has two electrons in its outer shell as it is in group 2 of the periodic table which it loses so its outer shell is full 28 and it has a charge of 2 as it still has 12 protons positive charges and now has only 10 electrons. 12 How many protons and electrons are there in the calcium ion.
Magnesium acquires a full octet by losing 2 electrons and emptying out its outermost shell. The valency shell means the outermost shell or orbit of an element which has valence electrons or outer electrons in it. How many electrons are in the outer shell of the magnesium on the periodic table.
When these electrons are lost a magnesium ion Mg 2 is formed. In a magnesium atom there are 12 electrons. The Valence shell of the element magnesium contains a total of 2 Valence electrons.
The element has a full innermost electron shell of two. Sulfur has 16 and Magnesium has 12. 14 How many electrons does calcium have in its outer.
Magnesium contains 2 electrons in its outer shell thus it has 2 valence electrons. The nucleus is composed of protons and neutrons. Since magnesium Mg has two extra electrons it looks around for elements which could use them.
Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. A magnesium ion has the same electronic structure as a neon atom Ne. The result is the Mg2 ion.
It is alkaline earth metal. 4 rows How many electrons are in the outer shell of magnesium. Magnesium has the same electron configuration in the ground state as aluminium which has four valence electrons.
A magnesium ion has the same electronic structure as a neon atom Ne. Hope this helps you good luck in Med-School. Magnesium has a total of 12 electrons.
Magnesium has a total of 12 electrons 2 in the innermost shell 8 in the second shell and two electrons in its valence shell third shell. Magnesium also has a filled shell.
How Many Electrons Does Magnesium Have Quora

The Number Of Electron Levels In A Magnesium Atom Is At Level
Magnesium Has 12 Protons How Many Electrons Are In Its First Energy Level Socratic
Comments
Post a Comment